The marine battery is a crucial component of a ship’s power system and serves as a vital source of power. The battery system not only supplies electricity to various lights on the ship, but also functions as an emergency power supply and a starter for the unit. Therefore, the proper use and maintenance of the marine battery is of utmost importance. However, some electricians may lack sufficient knowledge about the battery, and their expertise may be limited to routine usage. This can lead to a shorter lifespan of the battery.

1.Discharge time rate and battery capacity
1.1 Impact of discharge time rate on battery capacity
The capacity of a battery will differ based on the discharge time rate, which is the amount of time it takes for the battery to discharge completely. Table 1 illustrates how the battery’s capacity changes when discharged at different time rates. It is crucial for ship personnel to be aware of these variations in capacity in order to ensure the optimal use of the marine battery.
| Discharge Rate | Battery Capaicty |
| 10 hours discharge rate | 100% |
| 7.5 hours discharge rate | 97.7%% |
| 5 hours discharge rate | 83.3% |
| 3 hours discharge rate | 75.5% |
| 2 hours discharge rate | 61.1% |
| 1 hours discharge rate | 51.4% |
Tabel 1 The cpacity under the different discharge rate
When a battery is discharged, lead sulfate is formed on both the positive and negative plates, based on the principle of battery discharging.
On the negative pole plate, Pb-2e+H2SO4→PbSO4+2H+.
On the positive plate, PbO2+2e+H2SO4→PbSO4+2H2O.
However, this chemical reaction process is not instantaneous; it gradually occurs from the outside to the inside of the electrode plate. During high current discharge, the active substances on the plate surface (PbO2 on the positive plate and Pb on the negative plate) preferentially react with the electrolyte (dilute H2SO4) to produce PbSO4, which has larger crystal size than PbO2 and Pb. PbSO4 tends to block the porous material in the battery.
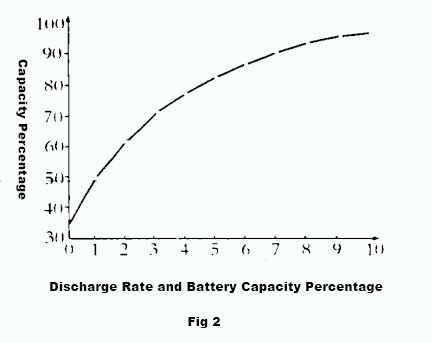
Additionally, due to the battery internal resistance , the voltage equation U=E-IR indicates that the terminal voltage U decreases as the discharge current I increases. The larger the discharge current, the faster the terminal voltage decreases. The higher the discharge current, the faster the terminal voltage drops, and the sooner it reaches the termination voltage. This allows for a shorter discharge time and results in a smaller capacity. Therefore, the battery capacity decreases with higher discharge currents. Figure 2 depicts the relationship between discharging current and capacity on a graph.
1.2 Temperature has a significant impact on battery capacity
When the temperature of electrolyte rises, the activity of electrolyte is enhanced and ionic state is easily formed, more electrons are gained and lost, and the battery capacity is relatively large. When the temperature of electrolyte decreases, on the one hand, the viscosity of electrolyte increases, the ion movement is subject to greater resistance, the diffusion ability is reduced, the replenishment of H2SO4 to the inside of the pole plate slows down, the utilization rate of active material deep in the pole plate decreases, and the capacity decreases; on the other hand, when the temperature decreases, the viscosity of electrolyte increases, the resistance of electrolyte increases, the internal pressure drop consumed in discharge increases, the end voltage decreases, the discharge time is shortened, and the capacity decreases. shorten the discharge time and reduce the capacity. Especially, low temperature and high current discharge has a greater impact on capacity. Figure 2 shows the relationship curve of electrolyte temperature to battery capacity.
Usually, the capacity of marine battery is tested under 25P condition with 10 hours current discharge, and the temperature change during discharge can be calculated by the following conversion formula.
When the temperature of the electrolyte increases, the activity of the electrolyte is enhanced, and the ionic state is easily formed. More electrons are gained and lost, resulting in a relatively large battery capacity. When the temperature of the electrolyte decreases, the viscosity of the electrolyte increases, and the movement of ions is subject to greater resistance. This reduces the diffusion ability and slows down the replenishment of H2SO4 to the inside of the pole plate. As a result, the utilization rate of active material deep in the pole plate decreases, and the capacity also decreases. Additionally, when the temperature decreases, the electrolyte viscosity increases, the resistance of electrolyte increases, the internal pressure drop consumed in discharge increases, the end voltage decreases, the discharge time is shortened, and the capacity also decreases. Low temperature and high current discharge have an even greater impact on the capacity. Figure 2 illustrates the relationship curve between electrolyte temperature and battery capacity.
Usually, the capacity of a marine battery is tested under 25℃ condition with 10 hours of current discharge. The temperature change during discharge can be calculated using the following conversion formula. K value is 0.008 .

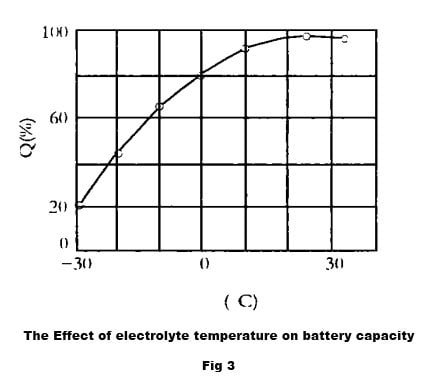

It is important to note that while increasing the temperature of the electrolyte can enhance the battery capacity, it can also cause damage to the pole plate and spacer, ultimately shortening the battery’s lifespan. Therefore, it is never acceptable to increase the working temperature to boost battery capacity in actual work. Typically, the electrolyte temperature of a marine battery should not exceed 35℃ to ensure optimal battery performance and longevity.
1.3 Effect of electrolyte concentration on battery capacity .
The electrochemical reaction in a battery is primarily determined by the active material on the positive and negative plates and the degree of ionizable H2SO4 in the electrolyte. Therefore, within the range of the actual electrolyte concentration, the battery capacity increases with an increase in concentration, especially during high-rate discharges.
However, increasing the electrolyte concentration can lead to larger viscosity, which is unfavorable to capacitance diffusion, and can also cause a rise in the internal resistance and self-discharge of the battery. Moreover, if the electrolyte concentration exceeds a certain limit, it can also accelerate the corrosion of the pole plate and spacer, leading to further damage.
1.4 The Effect of Electrolyte Purity on Battery Capacity
The purity of the electrolyte, which is made of a certain proportion of pure sulfuric acid and distilled water, has a significant impact on the battery capacity. This is because the electrochemical reaction in the solution is what causes the electrical energy input and output of the battery, and too many harmful impurities can greatly affect the battery’s working performance.
1.5 The Impact of Termination Voltage on Battery Capacity
The termination voltage of the battery is the voltage specified at the end of the battery discharge, and it varies depending on the discharge rate. Generally, at low discharge rates, the termination voltage is higher, while at high discharge rates, it is lower. This is because when the battery is discharged at low current, the electrochemical reaction inside the battery is more uniform, and as the discharge nears the end, most of the active material on the positive and negative plates has been transformed into lead sulfate (PbSO4). Since the volume of lead sulfate is larger than that of the original active material, the expansion of the active material can produce stress, causing the plates to bend or the active material to fall off, which can ultimately affect the battery life. Therefore, a higher termination voltage should be taken for low current discharge.
On the other hand, the termination voltage can be taken as a lower value for high current discharge, as the lead sulfate generated during this type of discharge is less, and the stress generated by the expansion is smaller. As a result, the electrode plate will not be damaged even when discharging to a fairly low voltage. Table 2 provides a comparison of discharge rate and termination voltage.
Table 2 Discharge rate and termination voltage(V)
| Discharge Rate (h) | 10 | 5 | 3 | 1 | 0.5 | 0.25 |
| Cut-off voltage | 1.79 | 1.76 | 1.74 | 1.68 | 1.59 | 1.47 |
- Incorrect usage conditions of boat batteries
2.1 Failure to charge in a timely manner
Boats typically have multiple battery groups, with two groups serving as backups for each other. One group is discharged while the other is charged, but sometimes the discharged battery group is not charged in a timely manner. The starting battery group is usually for diesel engines and may be dedicated or shared. Diesel engines require a high starting current, and after each start, the battery’s electric energy loss is supplemented by the diesel accompanying charging generator. However, the accompanying generator and regulator are prone to failure, and many boats will disconnect the accompanying charging generator to save trouble, resulting in the battery not being charged in time after starting the diesel engine, leading to excessive discharge. When the boat is undergoing repairs or is docked for a long time without any sailing tasks, the battery may be idle for an extended period of time, and regular battery inspections may not be conducted, resulting in some batteries being discharged due to self-discharge and not being charged in time.
2.2 Insufficient Charging
Most boats and cruise ships use the constant voltage charging method which is simple and easy to operate. The charging current can be automatically reduced from large to small, but the initial charging current is large, which can lead to high electrolyte temperature. Also, the late charging current is small and the power may not be sufficient. Many people tend to only charge the battery to half and think it is enough, but this causes the battery to be undercharged for a long time, which leads to a reduction in battery capacity as the active material on the pole plate is not completely restored.
2.3 Excessive Discharge
For electric starter diesel engines, the starter battery is mainly relied upon for starting energy. If the diesel engine is started for a long time or started too often with insufficient battery power, it can cause excessive battery discharge. Additionally, if battery discharge management is not properly maintained and the battery has reached its end of discharge mark but is still in use, it can cause excessive battery discharge.
These conditions can cause battery plate sulfation, active substance shedding, faulty self-discharge, and other failures, leading to a reduction in battery capacity and a shortened battery lifespan.
3. Solution for Marine Battery Maintenance
An effective capacity maintenance testing and management method for marine batteries has been a concern for many maintenance personnel. Comprehensive battery maintenance is a heavy workload for electrical engineers. The advanced and effective battery test equipment can reduce the intensity of battery maintenance works . KV Hipot provides the professional battery solutions .
KVBT-1500T Battery Internal Resistance Tester
KVBT-1500T Battery Internal Resistance Tester adopts an advanced AC discharge test method that integrates the advantages of AC injection method and DC discharge method. The principle is that the processor controls the adjustable load through D/A, allowing the battery to discharge to the load and resulting in a low-frequency signal through internal shunt, coupling, and filtering processing. Battery tester can accurately measure the voltage and internal resistance at both ends of the battery and use them to determine the battery capacity and technical state. Customers can choose the internal resistance test, voltage test, and capacity estimation of the battery according to their own situation. It can be used as the basis for matching the internal resistance of new batteries and can test the internal resistance of batteries before and after discharging to identify the real backward batteries. It has two operation modes: key operation and LCD touch, and can measure batteries in groups as well as single section measurement.
KVC-3800 Battery Charger Discharger
Battery Charger discharge integrates battery constant current discharge, intelligent charging, and activation into one machine for multiple uses. It reduces business costs, reduces maintenance personnel labor intensity, and provides scientific means of battery and UPS power supply maintenance testing for regular battery pack testing and backward battery re-life. According to the need for deep discharge and then charging, the battery pack can maintain a state of satisfaction at all times, extending battery life. This tester is a good assistant to the battery maintenance work





